Nils' Paper Online!
"Influence of Quinoidal Distortion on the Electronic Properties of Oxidized Divinylarylene-Bridged Diruthenium Complexes"
Nils Rotthowe, Jakob Zwicker, Rainer F. Winter
Organometallics, 2019, 38, 14, 2782-2799
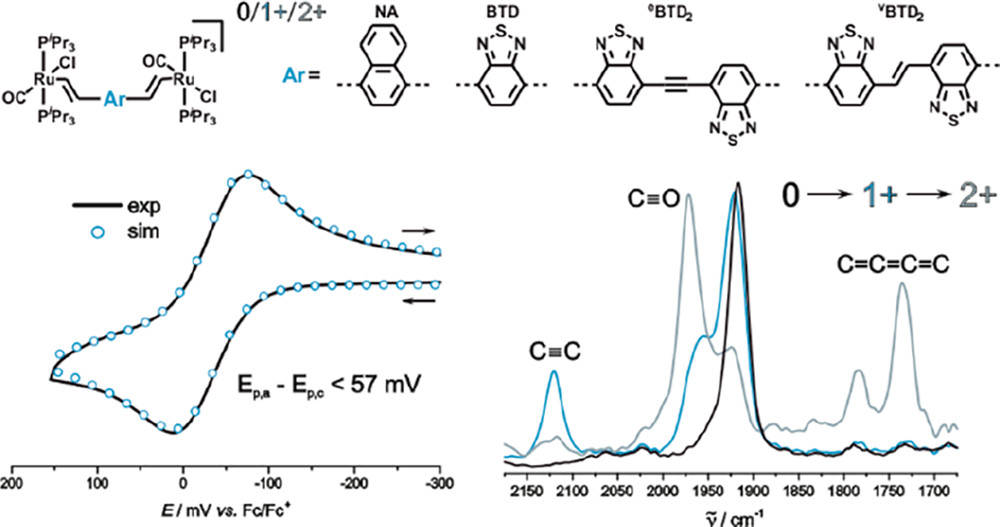
We present four new divinylarylene-bridged diruthenium complexes of the type {Ru(CO)Cl(PiPr3)2}2(μ-CH═CH–Ar–CH═CH) with naphthalene-1,4-diyl (Ru2NA), 2,1,3-benzothiadiazole (Ru2BTD), bis(benzothiadiazolyl)ethene (Ru2vBTD2), or bis(benzothiadiazolyl)ethyne (Ru2eBTD2) as the arylene units. The radical cations of the complexes with shorter arylene bridges are intrinsically valence-delocalized on the IR time scale. In contrast to phenylene-bridged [{Ru(CO)Cl(PiPr3)2}2(μ-CH═CH–C6H4-1,4-CH═CH)]2+ (Ru2-p-phen2+), the dioxidized forms of complexes Ru2BTD and Ru2NA are electron paramagnetic resonance (EPR) silent. Replacing the E-stilbenediyl linker of the complex {Ru(CO)Cl(PiPr3)2}2(μ-CH═CH–C6H4–CH═CH–C6H4–CH═CH) (Ru2vp-phen2) by vBTD2 or eBTD2 enhances electronic coupling and charge delocalization of their one-electron-oxidized mixed-valent forms. While the latter radical cations are still charge-localized species on the IR time scale, they are fully delocalized on the slower EPR time scale. As a token of a quinoidally distorted bridge, Ru2eBTD22+ features a characteristic IR stretch of a cumulenic C═C═C═C increment. For compound Ru2vBTD2, rearrangement to a quinoidal structure is manifested through potential inversion (i.e., the half-wave potential of the second oxidation is lower than that of the 0/1+ redox couple) in the CH2Cl2/NBu4BArF electrolyte. The BTD complexes are intensely colored in their neutral states and exhibit strong NIR absorptions of their radical cations as well as of their dioxidized forms.
https://pubs.acs.org/doi/10.1021/acs.organomet.9b00318
