Check out our 2023 paper on 1,4-Divinylphenylene-bridged diruthenium complexes
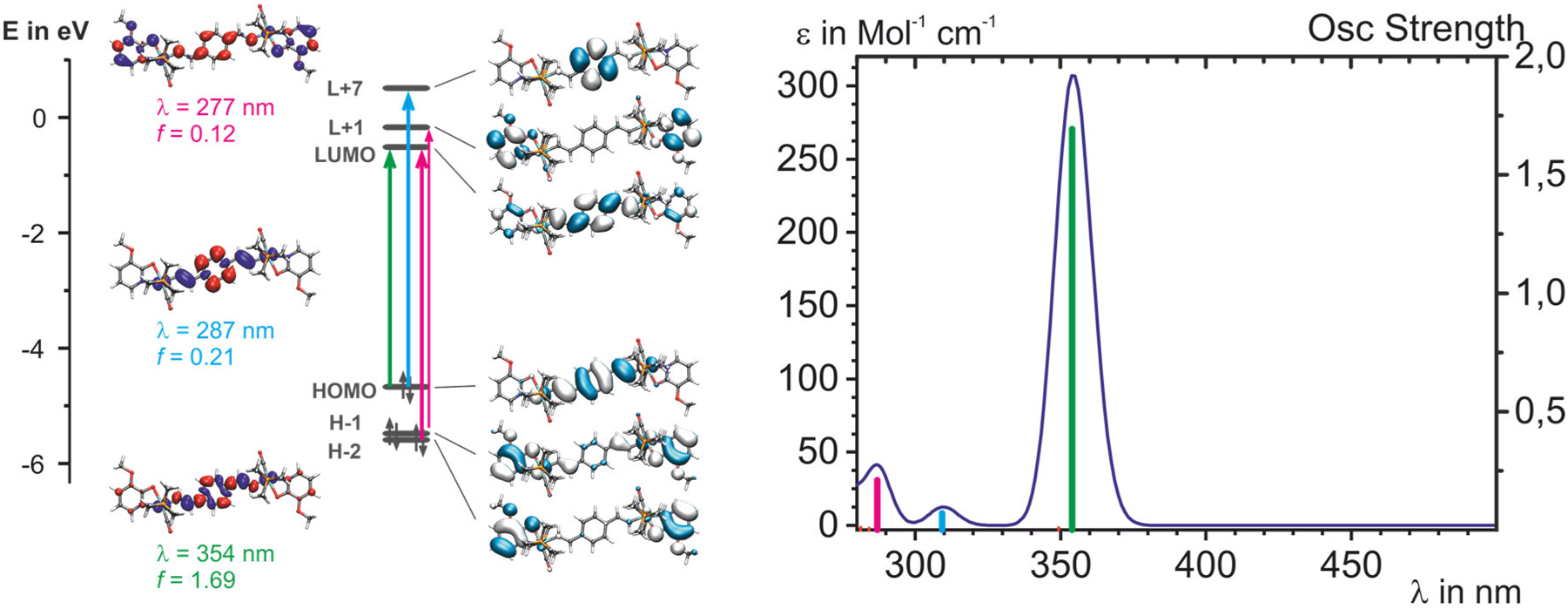
The study introduces four diruthenium complexes featuring divinylphenylene-bridged structures with N,O-chelating 2-hydroxypyridine and 2-hydroxy- or 8-hydroxyquinoline ligands. Through a combination of experimental techniques and (TD-)DFT calculations, the research reveals that the divinylphenylene bridge significantly influences redox processes, showcasing electron delocalization over the entire π-conjugated backbone with minimal involvement of the attached donor ligands.
Four divinylphenylene-bridged diruthenium complexes [{Ru(CO)(P i Pr3)2(L-κ 2 [N,O] – )}2(μ-CH=CH-C6H4-CH=CH-1,4)] (2a–2d) with N,O-chelating 2-hydroxypyridine and 2-hydroxy- or 8-hydroxyquinoline ligands are presented. They were studied by NMR spectroscopy, electrochemical methods and, in their neutral and oxidized states, by IR, UV/Vis/NIR and, if applicable, by EPR spectroscopy. The experimental studies are complimented by (TD-)DFT calculations. Our results indicate that the pyridine-olate complexes 2a,b exist as three isomers with a ratio of about 78:20:2 that differ with respect to the orientation of the N and O donors relative to the CO and alkenyl ligands in the equatorial coordination plane. Only the isomer with both imine N donors trans to the alkenyl ligand is observed for complexes 2c,d with quinolinato ligands. All complexes undergo two consecutive, chemically and electrochemically reversible one-electron oxidations at low potentials. Our results indicate strong contributions of the divinylphenylene bridge to the redox processes and an even delocalization of the electron hole and the unpaired spin density over the entire π-conjugated divinylphenylene diruthenium backbone with only minor involvement of the peripherally attached κ 2 [N,O] – donor ligands. The article is accesible free of charge under the following link.
