Research
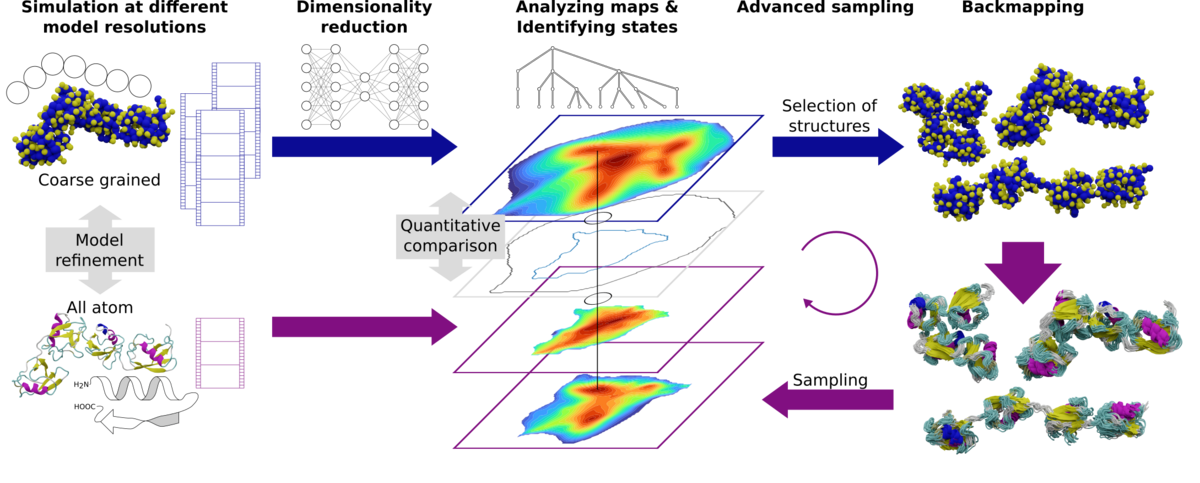
What we do
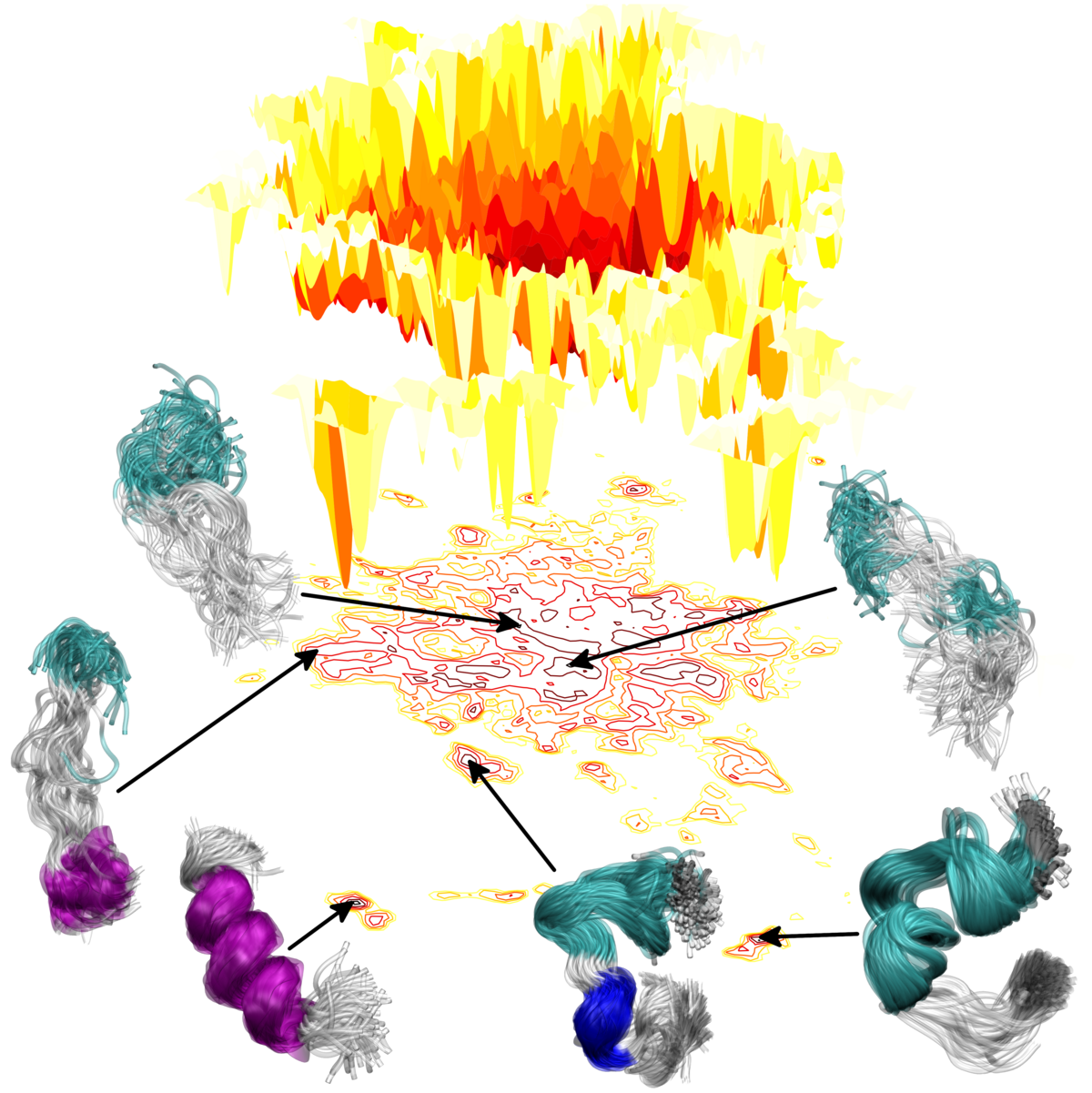
Our research lies in the field of classical simulation of biomolecular systems as well as biomimetic and synthetic nanomaterials. We develop hierarchical simulation approaches that systematically link models at several levels of resolution, for example combining an all-atom and a coarse-grained description. With such a multiscale approach we can address processes that require both chemical specificity as well as the sampling of long time and large length scales. In combination with ever-increasing computational power, these methods generate huge non-linear, high-dimensional datasets. Thus we employ concepts ranging from machine learning to network theory to extract information about structure and dynamics from these data.
Combining modern sampling and analysis methods, we investigate problems such as peptide folding, ligand binding, protein-membrane interactions, multi-domain systems, nanoparticle formation…
Current projects

EncoderMap
EncoderMap is our in-home developed tool for dimensionality reduction using a neural network autoencoder architecture. The newest release of EncoderMap comes with many quality of life improvements helping with featurization of MD data and introduces easy APIs to extend and customize the building, training, and reporting processes of the neural network. Find the documentation on: ag-peter.github.io/encodermap/index.html
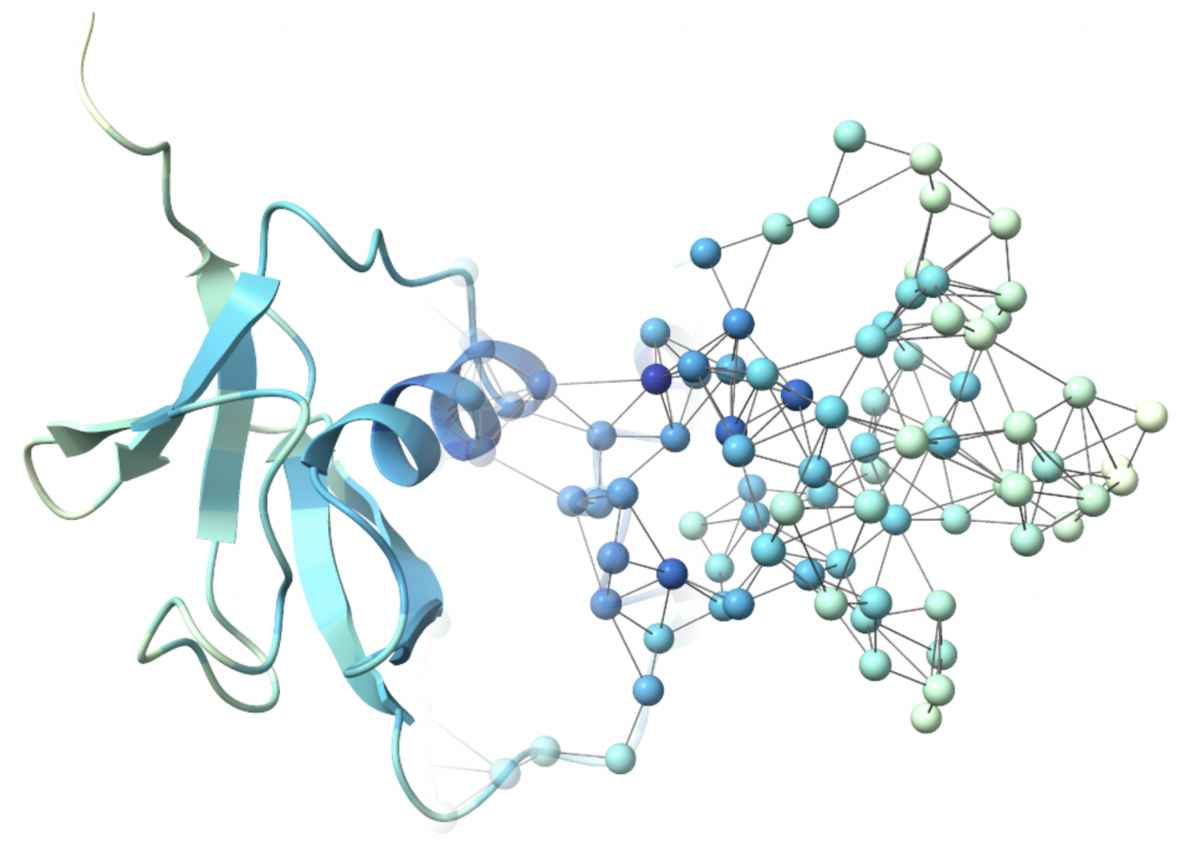
Understanding biomolecules using network theory
Biomolecules are complex dynamic systems governed by the interactions within them. Network theory offers powerful tools to describe and understand these interactions. By integrating network theory with molecular simulations and machine learning we want to gain insight into complex biomolecular processes on multiple scales, both in space and time.
Ubiquitin and ubiquitin-like conjugates
The protein ubiquitin didn't get its name by chance. With roughly 5% of the human genome encoding ubiquitin-ligases and an outstanding evolutional conservation from yeast to humans, ubiquitin is truly everywhere. What's even more, ubiquitin has been found to play key roles in genetic and proteinic cellular processes. While ubiquitylation of certain substrate proteins dooms them for degradation by the 26S proteasome, ubiquitylation of DNA nucleoproteins can silence genes and regulate DNA accessibility.
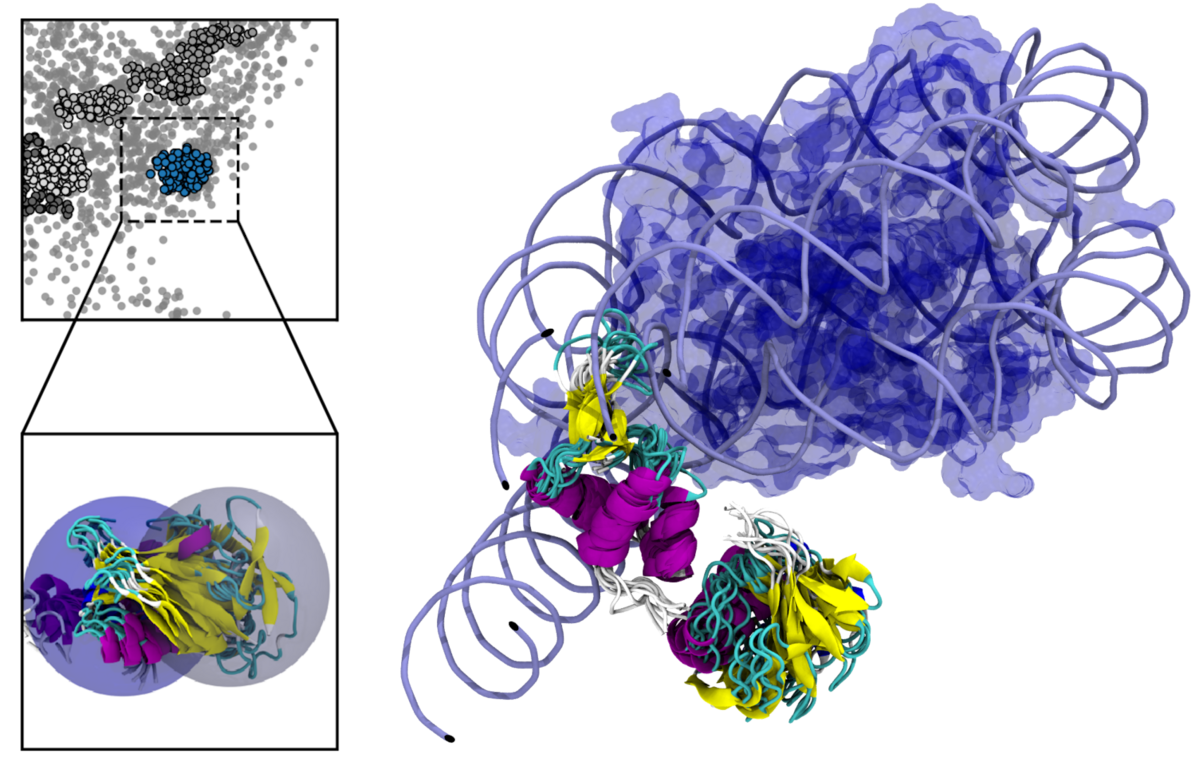
Modeling chromatin structure
A cell's DNA is packed as a nucleoprotein complex compising the DNA and histone proteins. These proteins govern the compaction of the genetic material into chromatin fibers and chromosomes. Various post-translational modifications can be observed for these proteins. Especially the ubiquitylation of the linker histone H1 poses as a system of research interest for us.
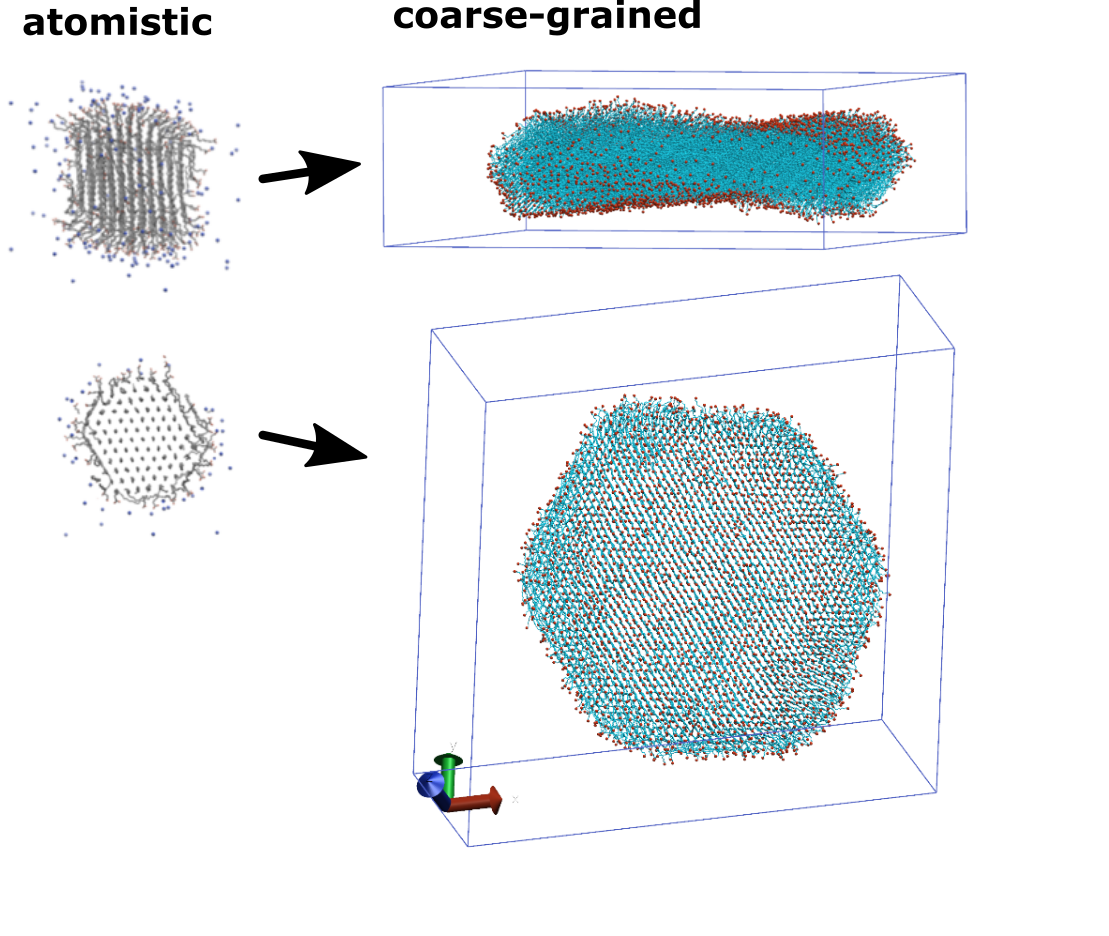
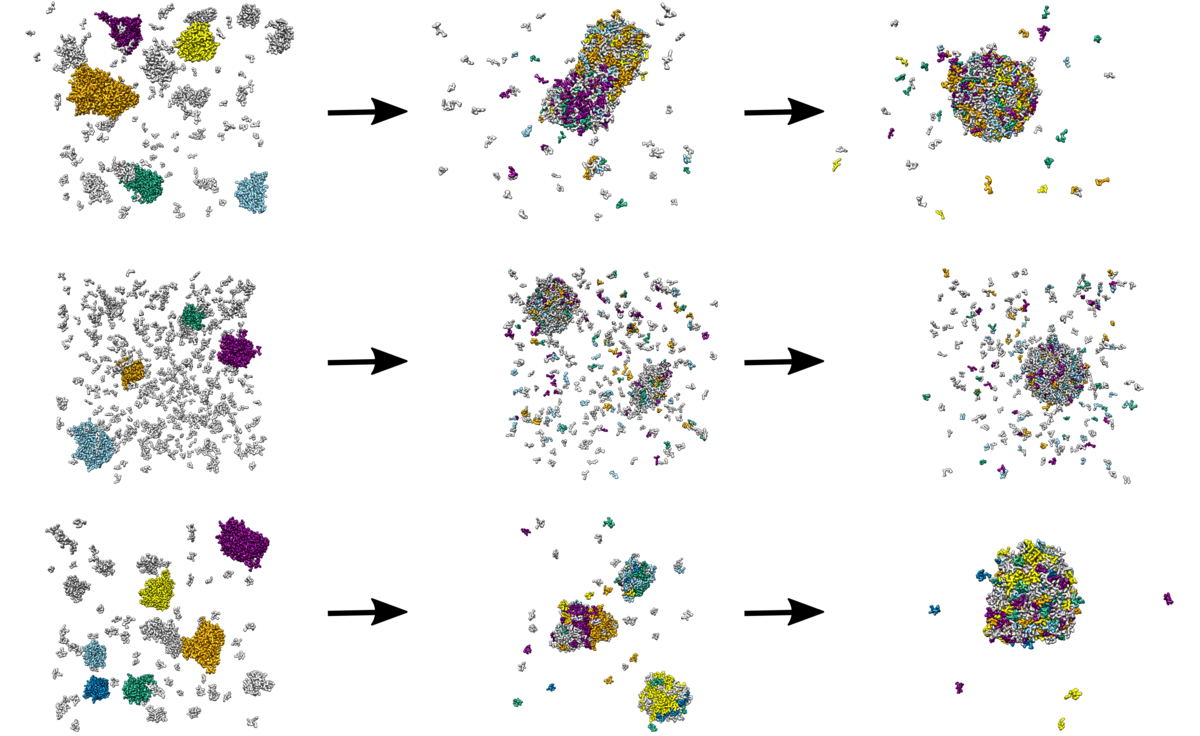
Polymer Nanocrystals
In cooperation with experimentalists of the research group of Prof. Dr. Stefan Mecking, nanocrystals made out of functionalized polymers are simulated on atomistic and coarse-grained resolution levels. Of particular interest are formation, stability and appearance of those nanoparticles, and differences when utilizing different functional groups.
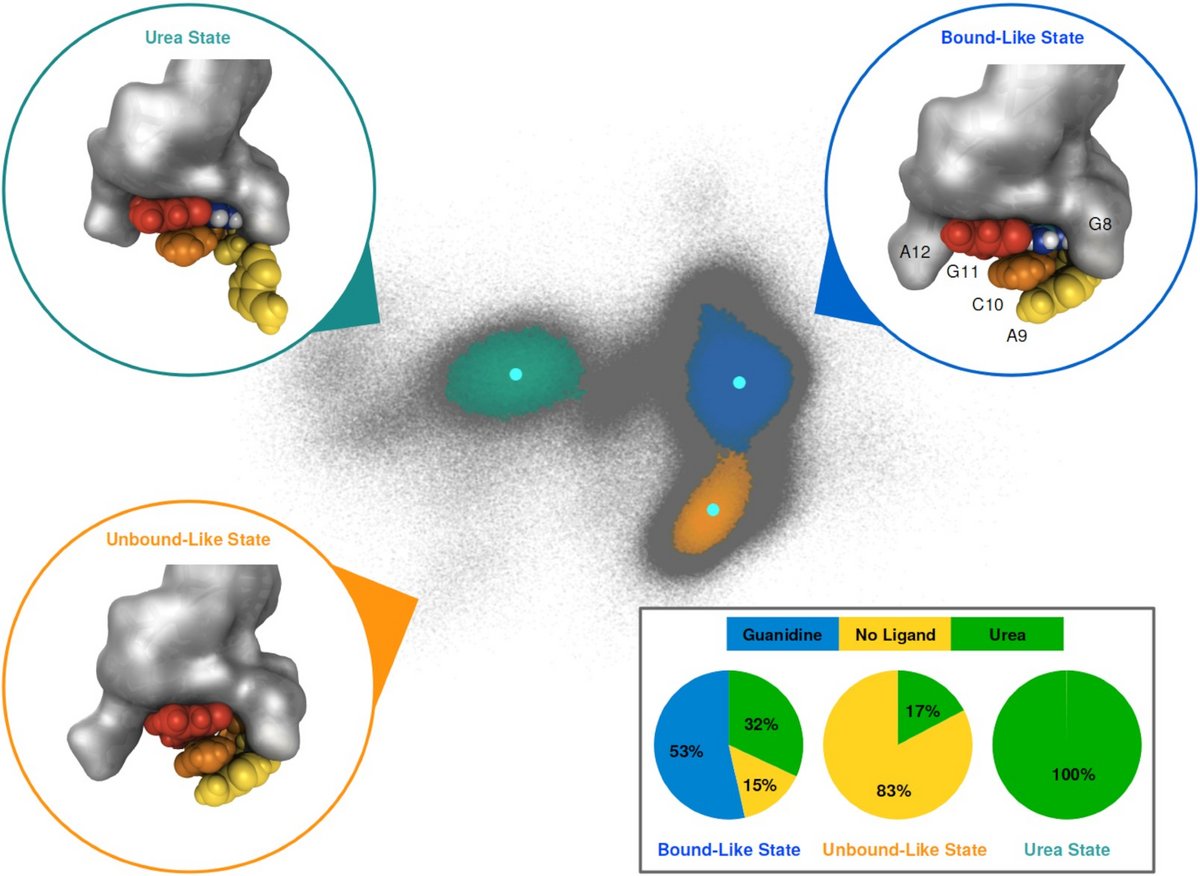
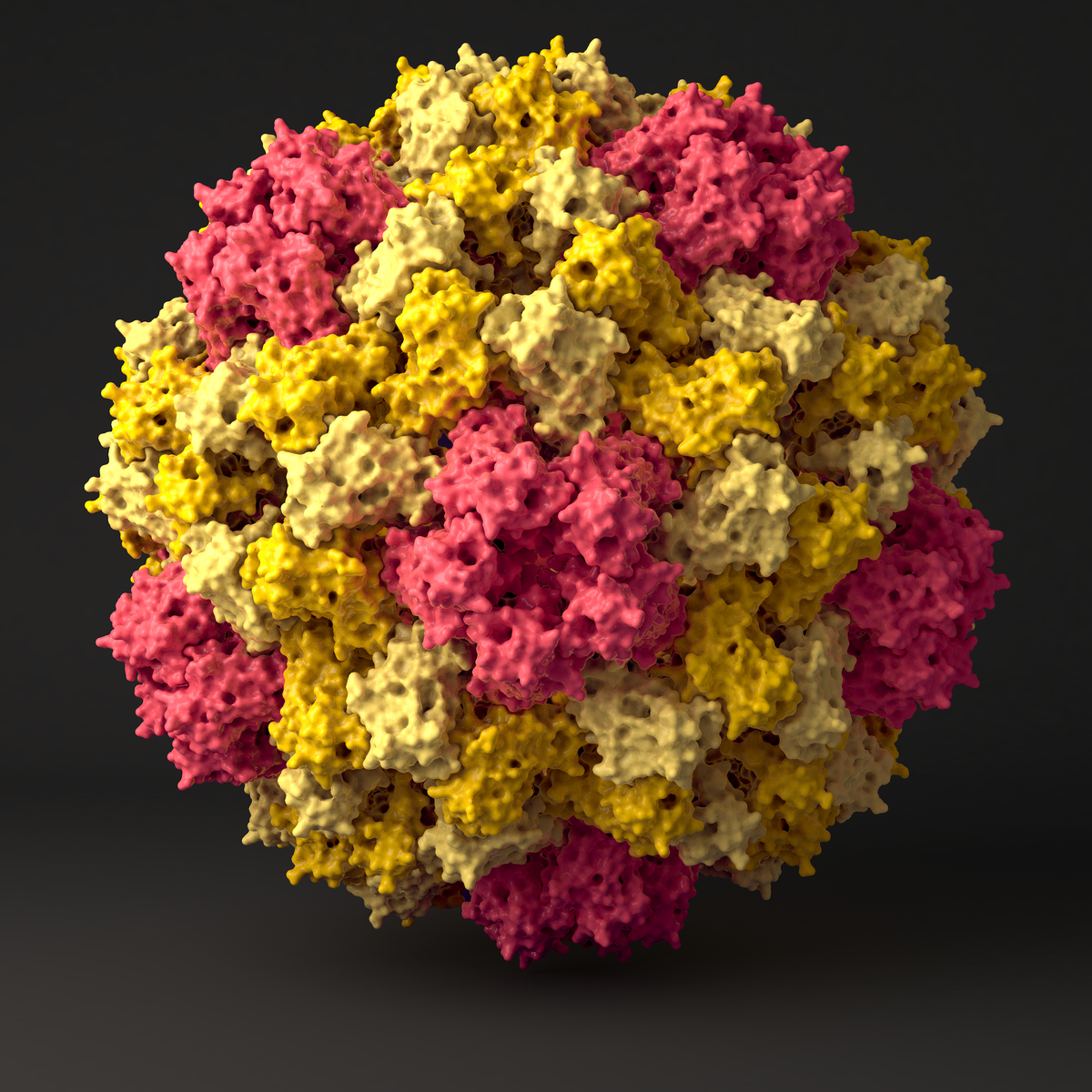
Riboswitches
Riboswitches reside in the 5′-UTRs of bacterial mRNAs and regulate gene expression upon interaction with small molecular ligands. We investigate ligand binding of a member of the guanidine sensing riboswitch family, the guanidine-II riboswitch (Gd-II) using extensive molecular dynamics simulations.
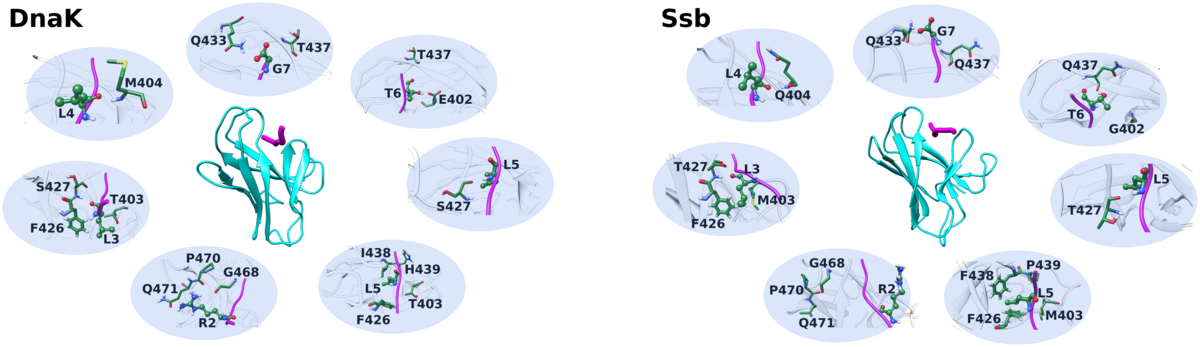
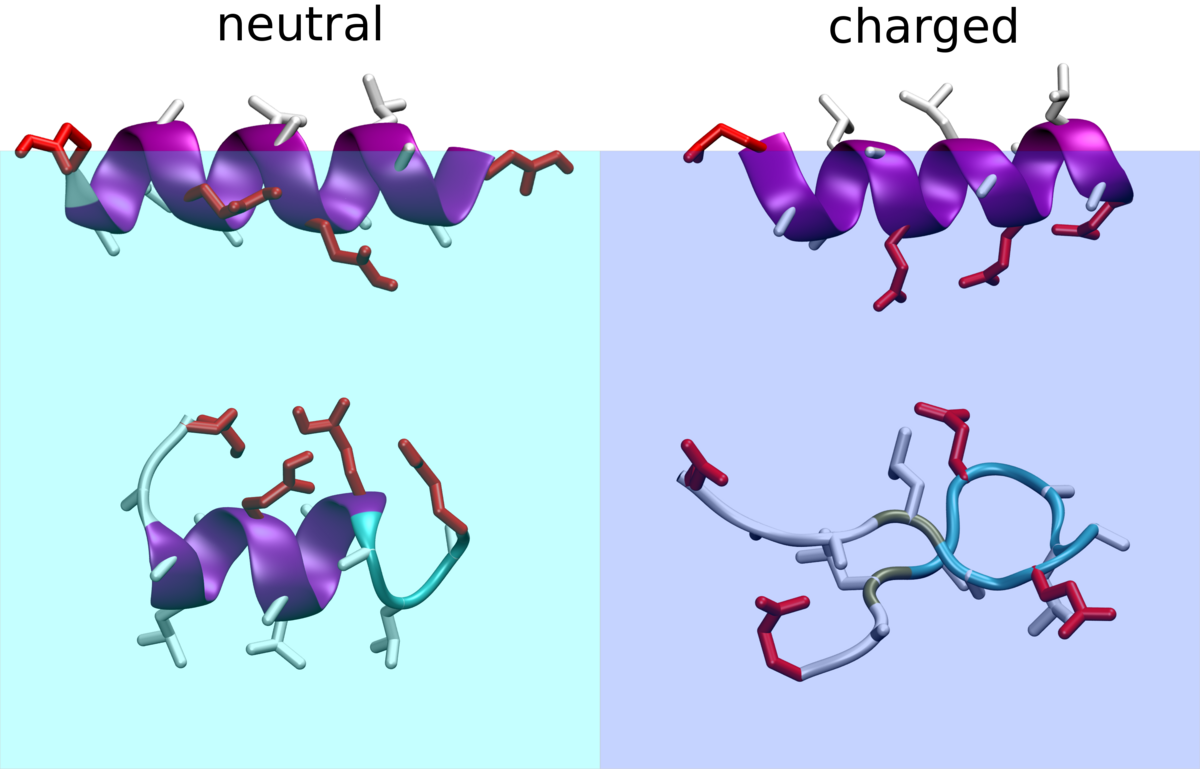
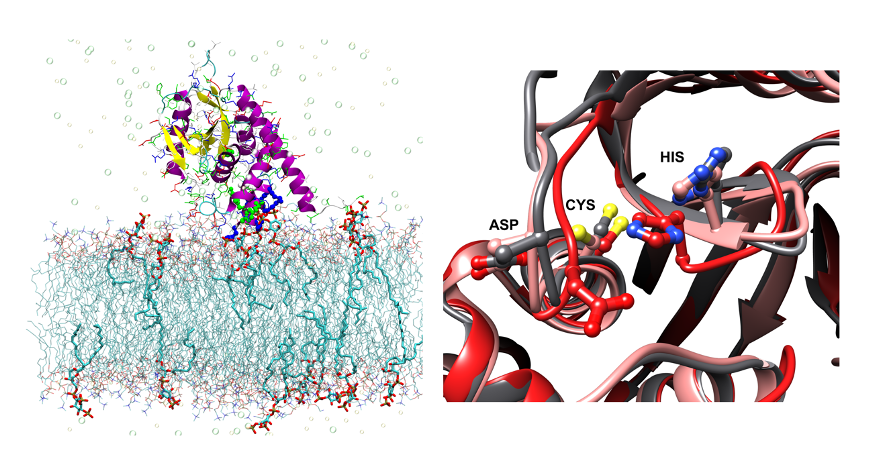
Plasma membrane-localized deubiquitylating enzymes
Studying protein membrane partitioning/interaction and its influence on the protein fold/function via molecular dynamic simulations at atomistic and coarse grained level.
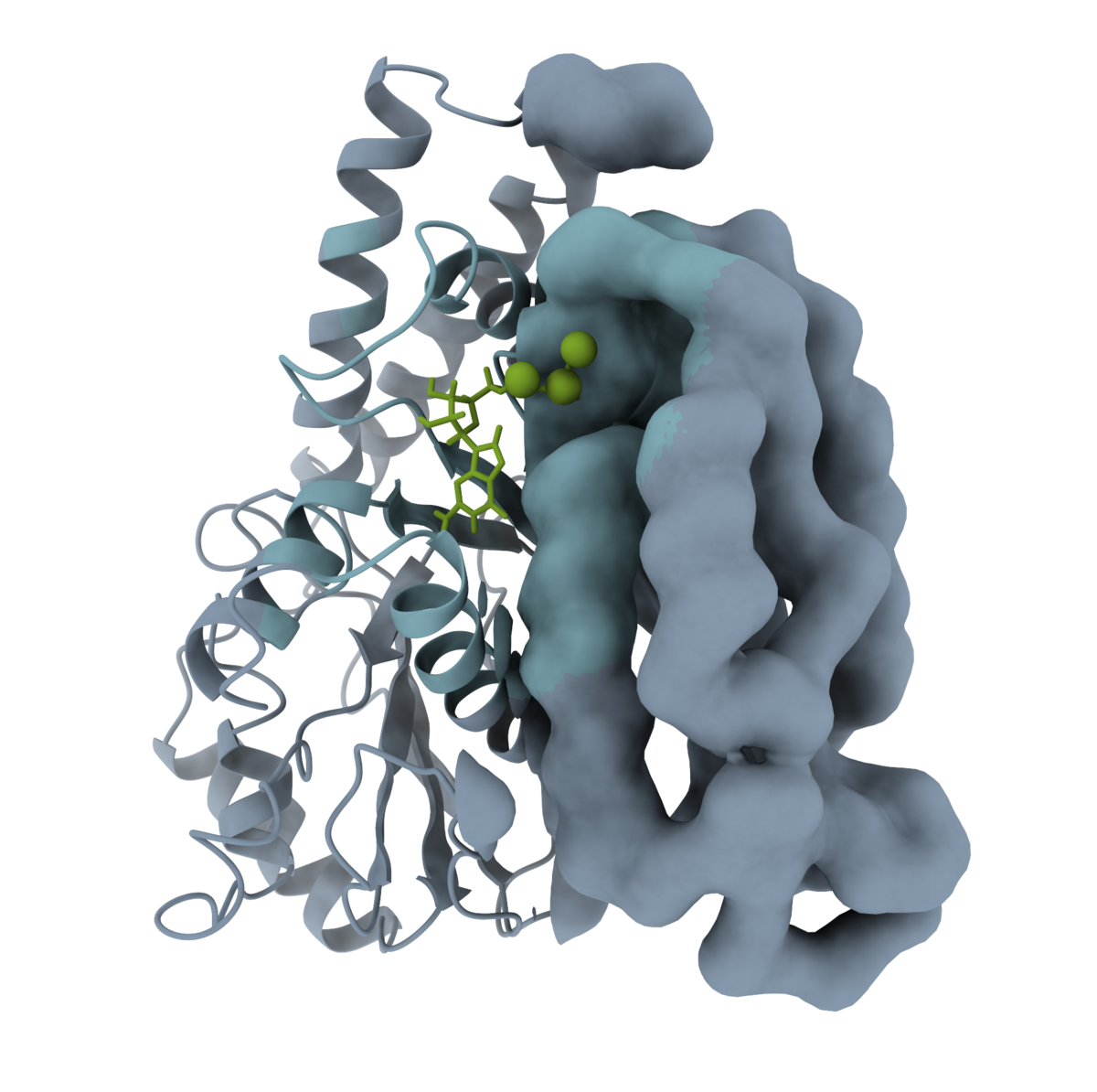
Modeling of microtubule dynamics
Microtubules, the cytoskeleton filaments of cells are widely used as cancer therapeutics targets due to their vital role in the cell division process. Recent studies that use cryo-EM and computational modeling approaches provide new perspectives on the intricacies of microtubule dynamics. We aim to complement the emerging views by simulating microtubule systems on the coarse-grained level.
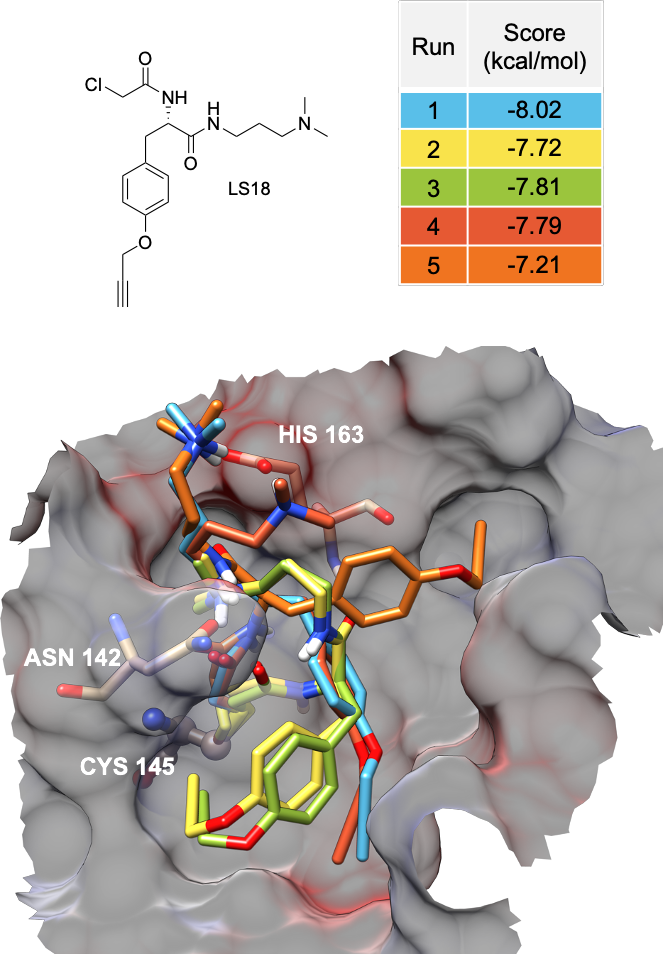
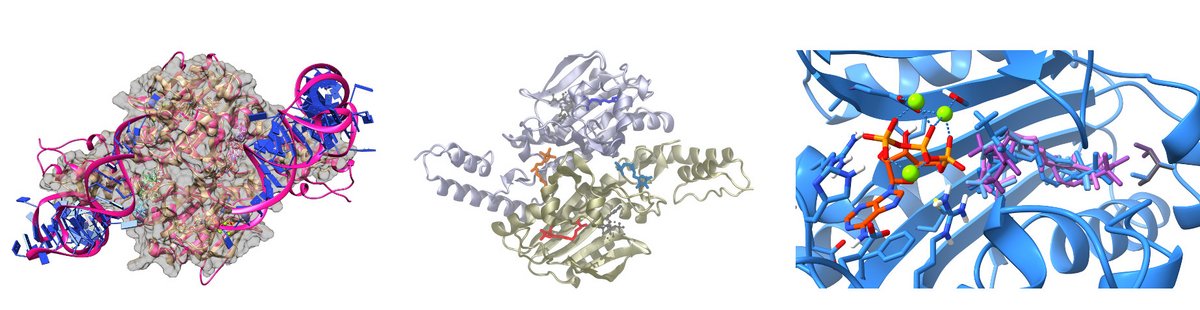
Augmenting EPR Data
Unraveling mechanistics aspects of protein function by combined approaches of elctron resonance spectroscopy an computational chemistry (modeling, docking, MD).
- Docking and MD studies are utilized to understand and visualize EPR-Data obtaned for ligands bound to Pyrrolysyl-tRNA Synthetase and develop a model for its alterating action.
- Combination of computational approaches and EPR-distance measurements are used to understand the role of local folding and the interaction within the ribosome-associated complex. Here especially the ribosome associated chaperone Zuotin with the Ribosome.
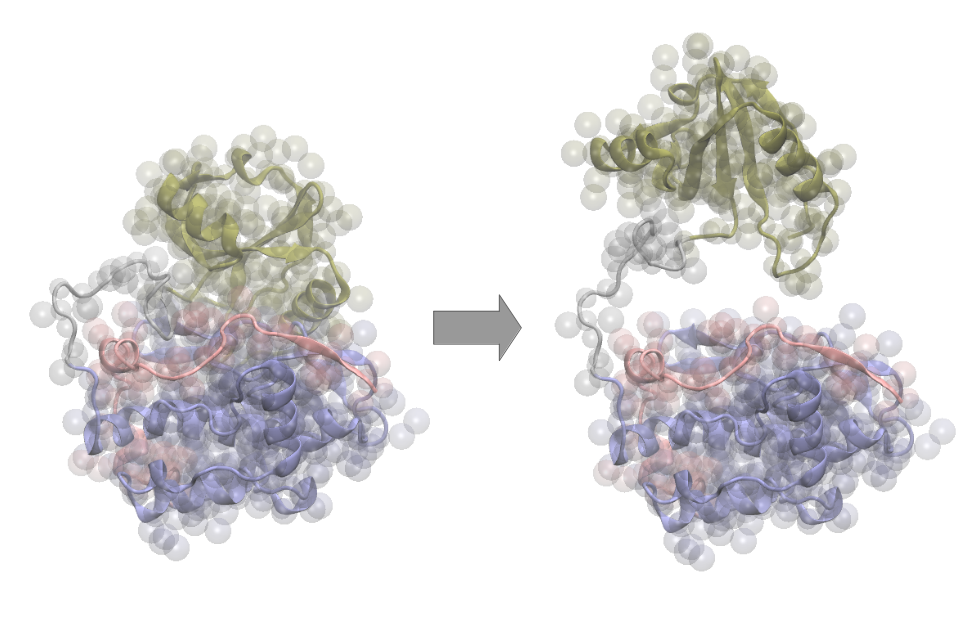
Muscle Protein Kinases
Specific protein kinases located in muscle fibers are postulated to act as mechanosensors. They are suggested to perceive the muscle sarcomere’s mechanical stretch and trigger signal pathways in response. To understand their mechanosensor activity, their conformational changes upon mechanical stretch need to be investigated. Multiscale molecular dynamics simulations are a promising tool in this regard, as they enable structural transformations to be studied on suitable time scales with atomistic resolution.