Muscle Protein Kinases
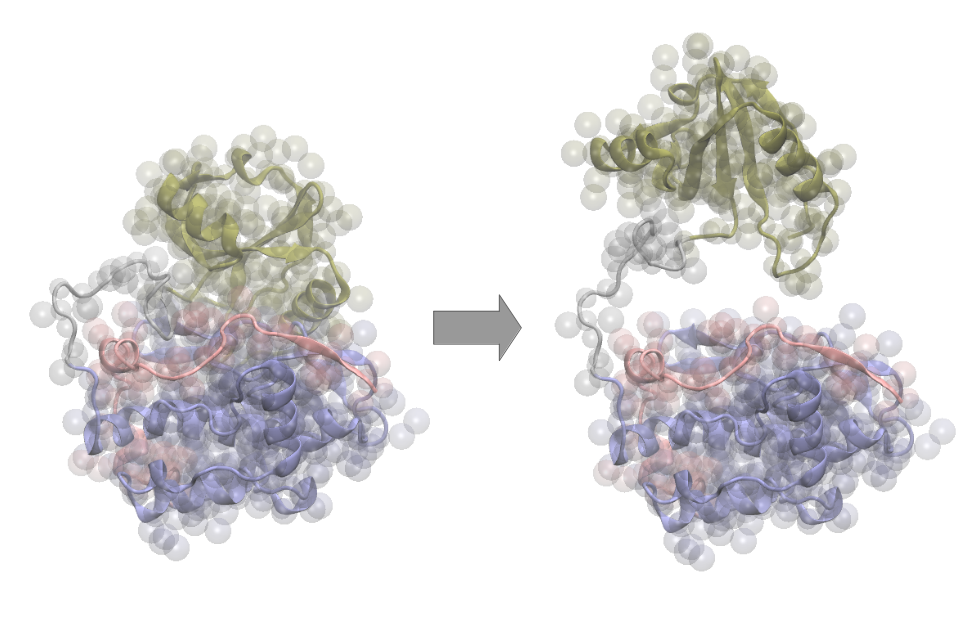
Specific protein kinases located in muscle fibers are postulated to act as mechanosensors. They are suggested to perceive the muscle sarcomere’s mechanical stretch and trigger signal pathways in response. To understand their mechanosensor activity, their conformational changes upon mechanical stretch need to be investigated. As these kinases are multidomain proteins, the alterations of their domain-domain interactions are of particular interest. Molecular dynamics simulations offer a great insight into structural transformations, hence they are very suited to study variations in interactions between protein domains. Especially the use of multiscaling techniques, meaning the combination of coarse-grained and atomistic MD simulations allows precise analysis on suitable time scales. To analyze the domain-domain interactions, both time series investigation and energy landscape examination are performed. Therefore a variety of parameters (e.g. residue-wise minimum distances) are investigated. Processing with for example machine-learning based dimensionality reduction algorithms and customized clustering methods allows the selection of energetically favored conformational states. These contribute to understand the overall functionality of the multidomain kinases.
