Plasma membrane-localized deubiquitylating enzymes
Plasma membrane-localized deubiquitylating enzymes
Studying protein membrane partitioning/interaction and its influence on the protein fold/function via molecular dynamic simulations at atomistic and coarse grained level.
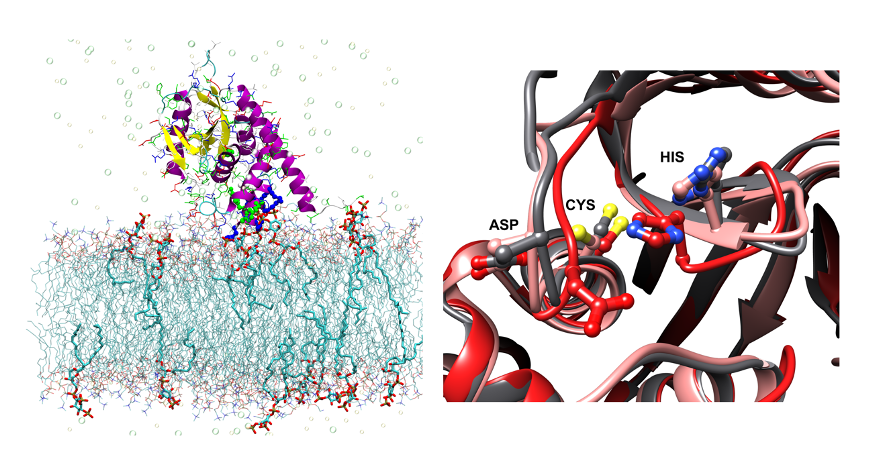
The abundance of plasma membrane-resident receptors and transporters has to be tightly regulated by ubiquitin-mediated endosomal degradation for the proper coordination of environmental stimuli and intracellular signaling. Arabidopsis OVARIAN TUMOR PROTEASE (OTU) 11 and OTU12 are plasma membrane-localized deubiquitylating enzymes (DUBs) that bind to phospholipids through a polybasic motif in the OTU domain. We could show that the DUB activity of OTU11 and OTU12 towards K63-linked ubiquitin is stimulated by binding to lipid membranes containing anionic lipids. In addition the DUB activity of OTU11 against K6- and K11-linkages is also stimulated by anionic lipids, and that OTU11 and OTU12 can modulate the endosomal degradation of a model cargo and the auxin efflux transporter PIN2-GFP in vivo. Our results suggest that the catalytic activity of OTU11 and OTU12 is tightly connected to their ability to bind membranes and that OTU11 and OTU12 are involved in the fine-tuning of plasma membrane proteins in Arabidopsis.
Lipid-mediated activation of plasma membrane-localized deubiquitylating enzymes modulate endosomal trafficking, K. Vogel, T. Bläske, M. K. Nagel, C. Globisch, S. Maguire, L. Mattes, C. Gude, M. Kovermann, K. Hauser, C. Peter, E. Isono, Nat. Commun., 13, 6897, 2022. [doi]