Fluorescence Lifetime Imaging Microscopy and Förster Resonance Energy Transfer
Förster Resonance Energy Transfer (FRET) is widely used to assess the proximity of two fluorophores. By labeling two interacting partners in close proximity (<10 nm) with donor and acceptor fluorophores, FRET can be used to investigate e.g. protein-protein interactions. We measure FRET via a reduction of the fluorescence lifetime of the donor fluorophore. As the fluorescence lifetime is a characteristic property of the fluorophore, it is generally independent of variations in fluorophore concentrations, scattering, or photobleaching and therefore allows for a robust and accurate detection of FRET.
We have developed a home-build wide-field frequency-domain fluorescence lifetime imaging (FLIM) microscope which allows us to monitor the fluorescence lifetime with high temporal and spatial resolution in biological samples. In the Zumbusch group, FLIM-FRET microscopy is applied to study post-translational modifications of proteins and ATP turnover, both in living cells.
Protein-specific visualization of post-translational modifications:
To date, more than 400 protein post-translational modifications (PTMs) are known. They were shown to have fundamental impact on the function, localization, stability, interaction and expression of proteins and further influence cell division, DNA transcription, and DNA repair. In order to study the biological roles of these PTMs, it is of great interest to visualize the modification of specific proteins in living cells.
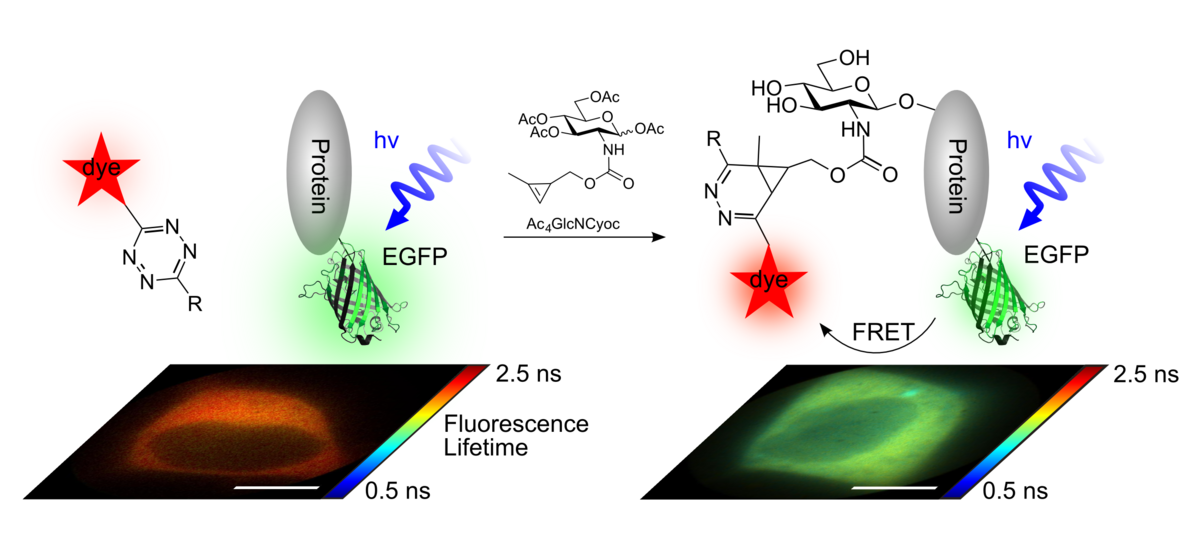
We achieve this goal by applying FLIM-FRET microscopy. To this end, the protein of interest is expressed as GFP-fusion protein inside mammalian cells. Additionally, cells are treated with chemical reporters for the corresponding PTM. In a second step, the PTM reporter can be covalently modified with a fluorophore by bioorthogonal ligation reactions. Then, FRET between the GFP and the PTM-anchored fluorophore is detected via the fluorescence lifetime of GFP.
We have developed the first approach to visualize the glycosylation of designated proteins inside living human cells. To this end, chemically functionalized monosaccharides (synthesized in the Wittmann group, University of Konstanz) were used as chemical reporters for glycosylation.
Published in:
F. Doll#, A. Buntz#, A.-K.-Späte, V.F. Schart, A. Timper, W. Schrimpf, C.R. Hauck, A. Zumbusch*, V. Wittmann, "Visualization of Protein-Specific Glycosylation inside Living Cells", Angew. Chem. Int. Ed., 55 (2016), 2262-2266.
Poly(ADP-ribos)ylation is a complex post-translational modification within which ADP-ribose is attached to proteins. The substrate is nicotinamide adenosine dinucleotide (NAD+). Poly(ADP-ribos)ylation is involved in DNA repair and gene regulation. In cooperation with AG Marx and the Bioimaging Center (both University of Konstanz), we have successfully imaged poly(ADP-ribose) turnover in real-time in living cells at DNA damage sites. Applying FLIM-FRET microscopy, we visualized the post-translational modification of selected target proteins with fluorescently labeled ADP-ribose.
Published in:
A. Buntz, S. Wallrodt, E. Gwosch, M. Schmalz, S. Beneke, E. Ferrando-May, A. Marx, A. Zumbusch "Real-time Cellular Imaging of Protein Poly(ADP-ribos)ylation", Angew. Chem. Int. Ed., 55 (2016) 11256–11260.
S. Wallrodt, A. Buntz, Y. Wang, A. Zumbusch, A. Marx "Bioorthogonally Functionalised NAD+ Analogues for In-Cell Visualisation of Poly(ADP-Ribose) Formation", Angew. Chem. Int. Ed., 55 (2016) 7660-7664.
Investigating ATP turnover
Adenosine triphosphate (ATP) is an universal energy source and involved in essential biological processes in every living organism. In cooperation with the Marx group (University of Konstanz), we study nucleotide turnover in the context of whole mammalian cells. Therefore, we use fluorogenic analogs of ATP. These bear a donor fluorophore attached to the phosphate groups and an acceptor fluorophore attached to the ribose of ATP. FRET between both fluorophores can only be observed, if the ATP analog is intact. Upon their hydrolysis, the FRET efficiency rapidly decreases.
We introduced these analogs via electroporation into mammalian cells and were able to monitor their turnover over time by measuring FRET.
Published in:
S. Hacker, A. Buntz, A. Zumbusch, A. Marx, "Direct Monitoring of Nucleotide Turnover in Human Cell Extracts and Cells by Fluorogenic ATP Analogs", ACS Chem. Biol., 10 (2015) 2544
