Mineral plastic" with great potential for the future
A new class of plastics has been inspired by nature and is easily degradable
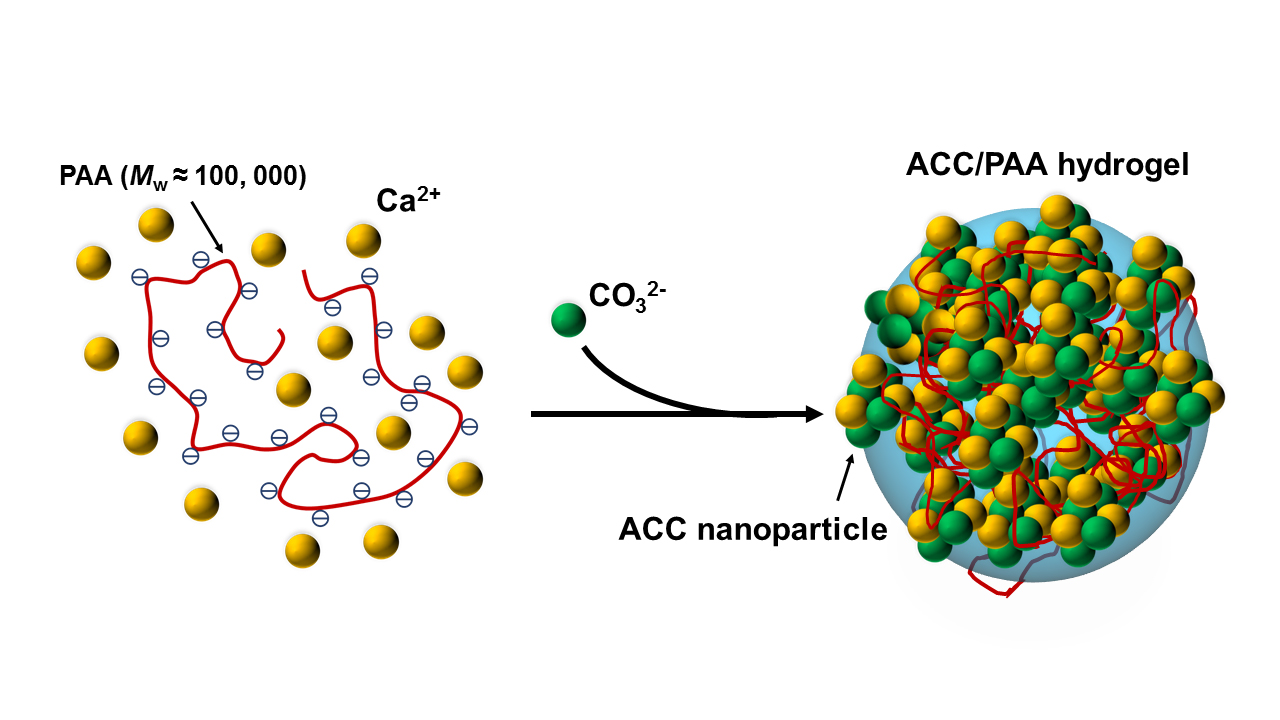
Conventional plastics are based on crude oil and cause problems for the environment as they are not degradable. The research group around Helmut Cölfen, professor of physical chemistry at the University of Konstanz, has now produced an entirely new "mineral plastic" whose structure copies biomaterials. The plastic is a so-called hydrogel that can be produced at room temperature from calcium carbonate and polyacrylic acid in water. The material can directly be recycled or transformed and is "self-healing" in its gel state. In the dry state, the material has the consistency of a crab shell and is pliable. The non-toxic plastic material might partly replace conventional plastics in the future and thus contribute to solving environmental problems. The paper has been published in the scientific journal "Angewandte Chemie" (DOI: 10.1002/anie.201602849).
Conventional plastics are usually not biodegradable, and the recycling process also requires energy. The research group from Konstanz used the guiding principle of "green chemistry" for the production of their mineral plastic. The process was inspired by mineralisation in nature, which is based on calcium carbonate. The hydrogel, which might replace plastics, consists of calcium carbonate nano particles. Polyacrylic acid is used to link these particles. The hydrogel can be produced without energy input at room temperature and is malleable and self-healing. Cracks, for example, will close again after applying a drop of water. Two separate components can be joined together in the same way. The gel can also be used as a temperature sensor, as it changes its colour when heated. Recycling the gel is no problem because it can be re-shaped without energy input. By adding water and a weak acid, such as acetic acid or citric acid, the gel will dissolve by releasing carbon dioxide. The residual polyacrylic acid is non-toxic.
"The production process of the hydrogel can directly be adapted by the industry, especially since the source materials are industrially produced at low cost," Helmut Cölfen explains. Once the material has dried, it takes on the distinctive qualities of plastic, as it is both durable and pliable at the same time. This makes it a suitable replacement for conventional plastics in dry applications, for example in electronic components. A further development of this substance might be cover material, which should not, however, affect the recycling process. The special swelling capacity combined with its hardness after drying makes the material suitable in building applications to fill cracks.
In contrast to biominerals, which are hard once they are finished, e.g. bones or teeth, the hydrogel is pliable. In addition to examining natural processes, the research group around Helmut Cölfen is now very interested in systematically changing the properties of such gels to produce other "mineral plastics" for specific applications. Future research projects will also consider possible medical applications for this new class of substance. The researchers will test other minerals as source material and they have planned to use polyaspartic acid as a potential cross-linking agent. This acid is completely biodegradable.
Original publication:
Hydrogels from Amorphous Calcium Carbonate and Polyacrylic Acid: Bio-Inspired Materials for "Mineral Plastics" Shengtong Sun, Li-Bo Mao, Zhouyue Lei, Shu-Hong Yu and Helmut Cölfen. Angewandte Chemie International Edition (DOI: 10.1002/anie.201602849).
