Interests
Our research addresses problems in materials chemistry, through polymers and nanoparticles designed to the purpose. Advanced catalytic methods are key to our approaches. These enable incorporation of functional groups, which are essential to achieve for example degradable and recyclable materials. They can also enable polymerizations in polar reaction media to afford unconventional morphologies.
Methods employed in our lab cover a rather broad span from fundamental studies of catalysis mechanisms, design of molecular catalysts, pressure reactor and polymerization processes (on a lab scale), to investigation of basic materials properties and processing. This is supported by an arsenal of methods for characterization of molecular and nanostructures of the products, comprising high temperature SEC, electron microscopy.
Current studies address four topical areas:
Materials from Nanocrystals
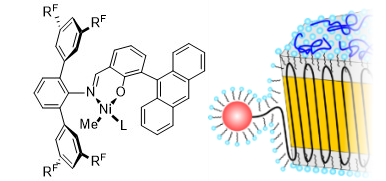
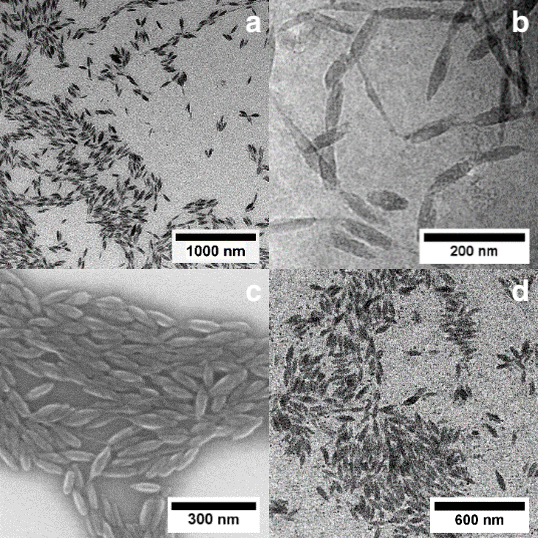
Water is certainly the most common dispersant encountered for nanoparticles, with their vast different facets. Also in technical applications, submicron particles are prepared on a large scale by free radical aqueous emulsion polymerization. By contrast to free-radical polymerization, catalytic processes allow for a control of microstructures but traditional olefin polymerization catalyts are incompatible with water. We have developed aqueous insertion polymerization, based on neutral late transition metal catalysts which yield polyethylene single crystals of nanoscale dimensions (e.g. 6 × 20 nm). With tailored catalysts that provide a defect free chain, nanocrystals with an unusually high degree of order are formed. This is attributed to their formation mechanism, in which the chains are deposited in an orderly fashion on the growth front of the nanocrystal as they are formed by insertion chain growth on the active sites (Figure 1) (Angew. Chem. Int. Ed. 2020, 59, 3258 & JACS 2013, 135, 11645). This leaves no opportunity for formation of entanglements.
Advanced catalysts enable a living polymerization in water, to yield ultra high molecular weight polyethylene. This is generated in the form of uniform size and shape single crystals (Figure 2) (Nature Commun. 2019, 10, 2592).
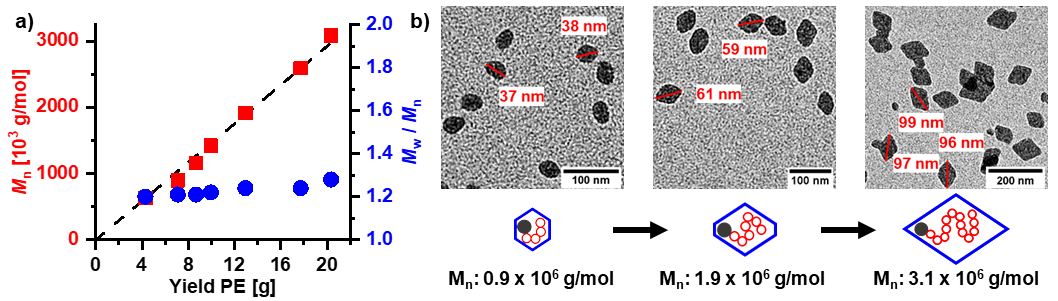
A key to accessing these ordered nanocrystals is a complete suppression of branch formation during polymerization. This is achieved by a catalyst design that considers secondary weak interactions of the chelating ligand with the active metal site (JACS 2018, 140, 1305). With strongly electron withdrawing substituents in remote positions, like pentaflurosulfanyl or perfluoroalkyl groups (Angew. Chem. Int. Ed. 2020, 59, 3258 & JACS 2017, 139, 13786), nanocrystals of ultra high molecular weight polyethylene are accessible. This concept also allows for generating monofunctional hyperbranched oligomers, which are of interest in their own right as lubricants or interface modifiers (JACS 2014, 136, 2078)
A focus of our ongoing work is now the generation of materials from these nanocrystals. For example, the notoriously unprocessable UHMWPE can be processed at ambient conditions from the nanocrystals by different methods, which enable new applications of this high performance material.
Polyethylene-like Polymers from Plant Oils
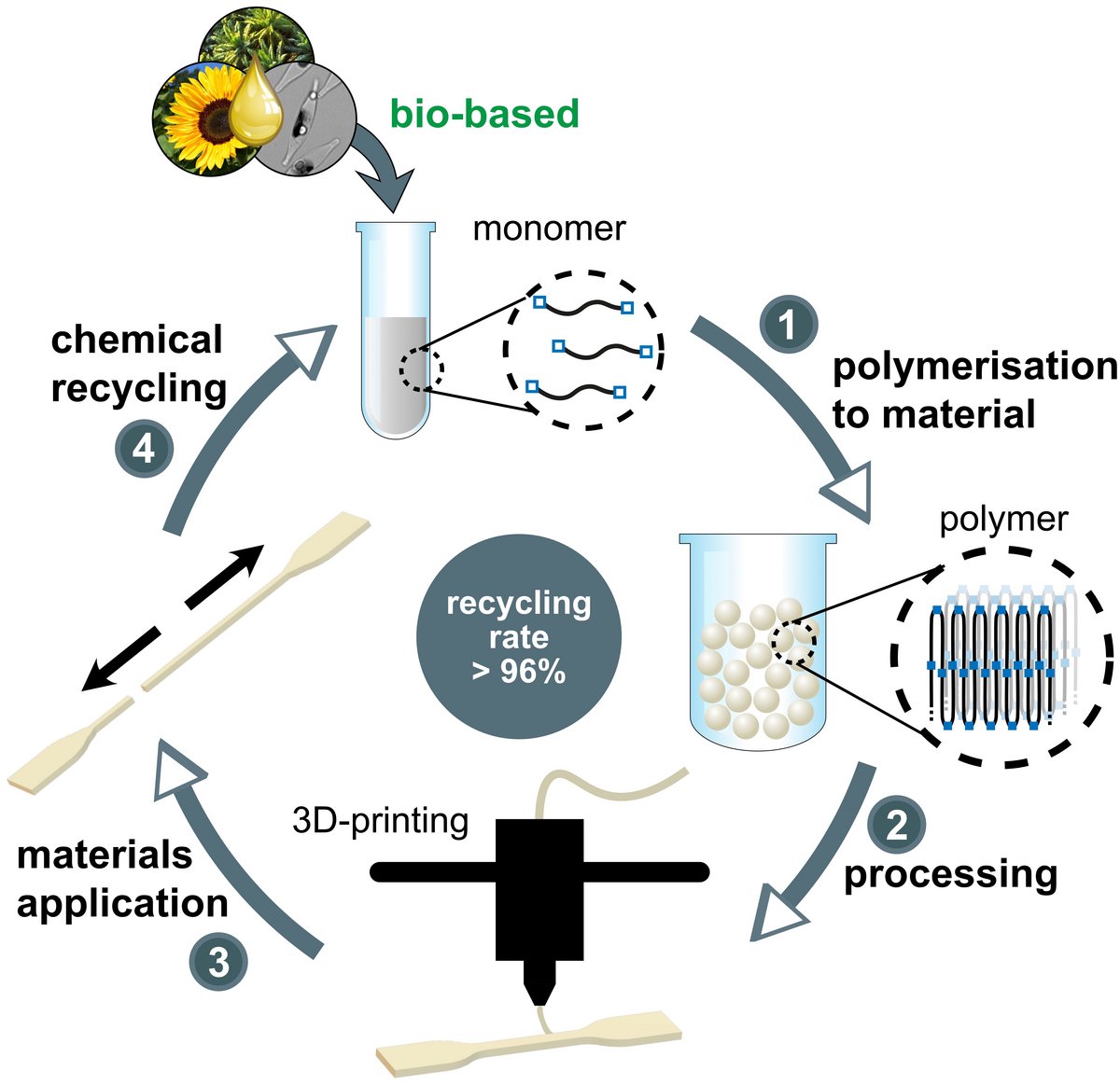
A preparation of materials from renewable feedstocks ideally carries their unique molecular structures over into the products, to provide them with useful materials properties. We have developed catalytic isomerization/functionalization reactions as a tool to generate long-chain linear monomers from plant oils, based on a mechanistic understanding (Angew. Chem. Int. Ed. 2010, 49, 4306, JACS 2012, 134, 17696 & JACS 2014, 136, 16871). By conversion of the double bond deep in the fatty acid chain to a terminal functional group, complete molecular utilization of the feedstock is achieved.
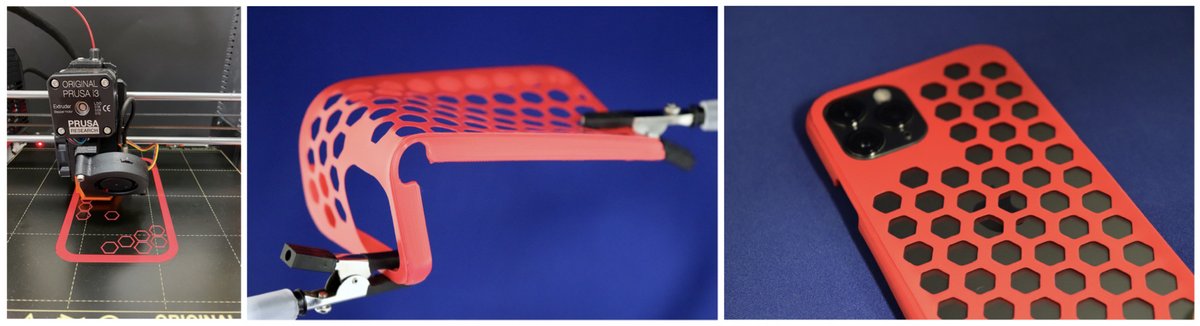
Polyesters or polycarbonates generated from such long-chain monomers can possess a polyethylene-like molecular and solid state structure, and their mechanical and processing properties match those of HDPE. At the same time the low density of in-chain functional ester and carbonate groups allows for a closed-loop recycling (Figure 4) with virtually quantitative efficiency (Nature 2021, 590, 423). Notably, these materials are also well suited for additive manufacturing (Figure 3). We are studying how these semicrystalline materials can be imparted with a hydrolytic degradability, in order to overcome the problem of long-term accumulation in, for example, marine environments (Angew. Chem. Int. Ed. 2023, 62, e202213438).
The polyethylene-like character of such polymers also enables an ordering of ionic materials on very short length scales due to microphase separation (JACS 2020, 142, 857 & JACS 2021, 143, 16725). These have potential as stable and directional electrolytes for energy applications.
Concering the sourcing of monomers, algae oils or waste oils as a feedstock do not consume arable land for their production, and microalgae can grow very rapidly. By means of catalytic isomerization/functionalization approaches both, otherwise difficult to access long-chain difunctional monomers (Angew. Chem. Int. Ed. 2014, 53, 6800), as well as today's petrochemical industries basic building blocks (Angew. Chem. Int. Ed. 2023, 62, e202219222) can be generated from alternative feedstocks. To facilitate the overall extraction and catalytic upgrading, we have integrated both using supercritical extractant in a flow process. Ongoing research efforts address catalytic processes that are compatible with the entire cell constituents, and can be integretated directly into living microalgae (Angew. Chem. Int. Ed. 2022, 61, e202211285).
Luminescent Nanoparticles
Conjugated polymers have been studied intensely for decades. In the form of nanoparticles, however, luminescent semiconducting polymers have achieved attention only recently (Chem. Rev. 2010, 110, 6260). Triggered by initial studies of inkjet-printable aqueous polyacetylene dispersions (Angew. Chem. Int. Ed. 2006, 45, 6314), we developed miniemulsion step-growth polymerizations to color-tunable bright conjugated polymer nanoparticles (JACS 2009, 131, 14267). Proof of principle of cell imaging including single particle observation and two-photon detection was demonstrated (Biomacromolecules 2010, 11, 2776). More recently, size dependent uptake was also studied (Macromolecules 2015, 48, 483). To provide precise microstructure conjugated polymers as heterodifunctional building blocks for e.g. amphiphilic block copolymer nanoparticles (Figure 5), we developed a living step-growth protocol starting from highly unsaturated palladium species (JACS 2013, 135, 1148). With endgroups like amines and phosphonates, these can also serve as 'stabilizers' directly in the high temperature synthesis of inorganic quantum dots, to yield hybrid organic/inorganic semiconductor nanoparticles (Adv. Funct. Mater. 2014, 24, 2714).
Heterodifunctional molecules with precise endgroup functionalization and tailored bandgaps are of interest as interface modifiers to study charge transfer between bulk inorganic (electrode) and organic semiconductor phases. Likewise in cooperation with colleagues from Physics, in the SFB767, hybrid particles with tailored interactions between the organic semiconductor shell and inorganic core were pursued as probes for fundamental studies of ultrafast single photon phenomena (Nano Lett. 2018, 18, 5396 - 5400). We have also been able to establish a reliable protocol for the generation of polyfluorene ellipsoids with narrow distributions and high aspect ratios (Angew. Chem. Int. Ed. 2017, 56, 6147 Figure 4). By means of ESR, conformations of moleclues in the particles could be accessed (JACS 2020, 142, 1952 - 1956).
Polar Monomer Copolymerization
Ever since the seminal discoveries of olefin polymerization by Ziegler and Natta - perhaps most instrumental in initiating our 'plastics age' - an incorporation of polar vinyl monomers has remained a challenge. It was not until forty years later that a first example of insertion polymerization of a polar vinyl monomer, in this case an acrylate was reported (JACS 1996, 118, 267 & JACS 1998, 120, 888). Highly branched amorphous materials were obtained here. More recent developments with a neutral unsymmetric coordinated Pd(II) catalyst have enabled the incorporation of many difficult polar vinyl monomers, including acrylic acid (JACS 2010, 132, 17690) and vinyl chloride (Angew. Chem. Int. Ed. 2013, 52, 3963). Even a homopolymerization of acrylate via an insertion mechanism could be demonstrated (JACS 2009, 131, 422), and approaches to address the regio- and stereoselectivity of the insertion steps - which requires a different concept than the well known space filling approach - was outlined (PNAS 2011, 108, 8955 & JACS 2013, 135, 1026). A major limitation are significantly reduced copoly-merization activities compared to polymerization in the absence of polar comonomers. Mechanis-tic studies have provided a quite comprehensive picture of the origin of this effect. Coordination of polar groups of the free monomer, and in particular of repeat units already incorporated in the growing chain blocks coordination sites reversibly and hinders chain growth (JACS 2010, 132, 4418 & JACS 2012, 134, 1010). Beyond these mechanistically oriented studies, we have provided first examples of specifically accessible materials with a direct synthesis of polar-substituted saturated polyolefin elastomers (Adv. Funct. Mater. 2014, 24, 387) and impact-improved polyamides (Macromol. Mater. Eng. 2018, 303, 1700276 Figure 6).
Ongoing and future research address the decisive problem of slow chain growth. One approach is a selective rapid secondary insertion after an incorporation of polar vinyl monomers by virtue of an appropriate second olefinic function in the monomer (JACS 2015, 137, 2836). A alternative different approach touching upon this problem are combinations of apolar olefin insertion chain growth and free-radical growth of polar monomer segments involving unpaired electron metal intermediates (ACS Catal. 2019, 9, 2760).
By incorporation of carbon monoxide in (controlled) free-radical polymerization, photodegradable polyethylenes could be achieved (Nature Commun. 2020, 11, 3693 & JACS 2022, 144, 15879). This provides branched keto-modified chains, optionally endowed additionally with in-chain ester groups. Catalytic copolymerization with Ni(II) catalysts enables the generation of linear keto-polyethylenes with isolated in-chain groups, a long-sought goal (Science 2021, 374, 604 & JACS 2022, 144, 15111). These preserve the processability and mechanical durability of HDPE, but endow the material with photodegradability that can reduce its persistency.
